Introduction
In recent years, several antibody-drug conjugates have received approval for the treatment of gynecological cancers, adding powerful tools to clinicians’ armamentarium. With these new approvals come new adverse events that require identification and management for patients to continue therapy safely, particularly ocular toxicities.
Although some oncology practices have experience with ocular side effects, particularly those treating breast cancers, melanomas, and lung cancers using taxanes, BRAF/MEK inhibitors, or platinum analogs, other practices may be unfamiliar with the spectrum of events. Given that ocular toxicity from vedotin-conjugated agents will affect half or more of prescribed patients, it is vital to understand what might manifest, what side effects to discuss with patients, and how to manage ocular toxicities effectively.
Why are vedotins in antibody-drug conjugates?
Vedotins are the anti-cancer agents or the drug component linked to the cell-targeting antibody of antibody-drug conjugates. They are the payload that will be internalized into the cell to wreak havoc on its functions.
Payloads are similar to chemotherapy, but their incorporation into a targeted conjugate was designed to make them safer and more effective against cancer cells without causing significant collateral damage to normal cells. They are toxic, stable molecules designed to kill upon internalization, with subsequent release of the linker.
Monomethyl auristatin E (MMAE) is the vedotin component, a microtubule toxin or microtubule disrupting agent. Vedotins inhibit the cell from correctly organizing tubulin, which is required for progression through the cell cycle for cell replication. They bind to tubulin molecules, arresting cells in phases of the cell cycle, eventually leading to cell death.
This is effective against cancer cells because poisoning tubulin operations prevents replication. Unfortunately, off-target effects impact normal cell types that require replication and cell turnover as a normal process.
Why are ocular toxicities associated with antibody-drug conjugates?
Any antibody-drug conjugate that impacts tubulin, or more broadly, cell replication, can cause indirect damage to vulnerable cells. The result is similar to chemotherapy impacting hair follicles, the lining of the intestines, red blood cells, and white blood cells. Antibody-drug conjugates are simply chemotherapy-like toxins attached to antibodies that release them inside cells. Each antibody carries multiple toxins.
Tubulin dynamics are required for normal cell replication to occur. Cell types that turn over as part of their normal functioning are vulnerable bystanders of the toxins. Therefore, the most common ocular structures affected are the lacrimal gland (dry eye), cornea (keratitis/perforation) and conjunctiva (dry eye, conjunctivitis, ulceration and symblepharon).
Examples of FDA-approved antibody-drug conjugates with vedotins include brentuximab- vedotin, enfortumab-vedotin, and tisotumab-vedotin. However, with antibody-drug conjugates, it is important to note that other drugs like datopotamab-deruxtecan and mirvetuximab-soravtansine also have ocular toxicities as their most notable concern. These vary slightly in severity and type from the vedotins, but impact a significant percentage of patients.
There are two other factors contributing to ocular toxicity with tisotumab-vedotin. The targeting antibody is against tissue factor, which is also expressed on the surface of conjunctival cells. In addition, the linker’s stability delays the split of the toxin from the antibody. The combination of the prolonged release causes the antibody-drug conjugate to remain in circulation longer, and systemic retention exacerbates the side effects.
How common are ocular toxicities associated with antibody-drug conjugates?
As many as 50% to 60% of patients will experience ocular toxicities during treatment with antibody-drug conjugates. Most of these are mild to moderate, not impacting quality of life and the ability to continue treatment. Less than 10% of patients reach grade 3 or higher.
The median time to onset in patients is 1.3 months for ocular toxicities to appear. However, they have also been reported up to 10 months after treatment initiation. These accounted for 5.0% to 35.0% of patients requiring a delay in treatment or a dose reduction.
In clinical trials, mirvetuximab-soravtansine treated patients showed blurred vision (40.8-42.0%), keratopathy (29.0-32.5%), and dry eye (25.0-28.0%). Patients treated with tisotumab-vedotin experienced conjunctivitis (red eye) (26.0-39.0%), dry eye (13.2-23.0%), and keratitis (Superficial punctate keratitis and ulcerative keratitis) (4.0-15.6%) and symblepharon (scarring of the conjunctiva). Corneal perforation was rare.
In general, the discomfort and pain that may result from ocular toxicities also contribute to patients desiring to discontinue treatment. Both prophylactic and reactive treatment are necessary when prescribing antibody-drug conjugates. Preventative measures reduced the incidence of conjunctivitis from 56% to 29% in a clinical trial.
How can clinicians manage ocular toxicities associated with antibody-drug conjugates?
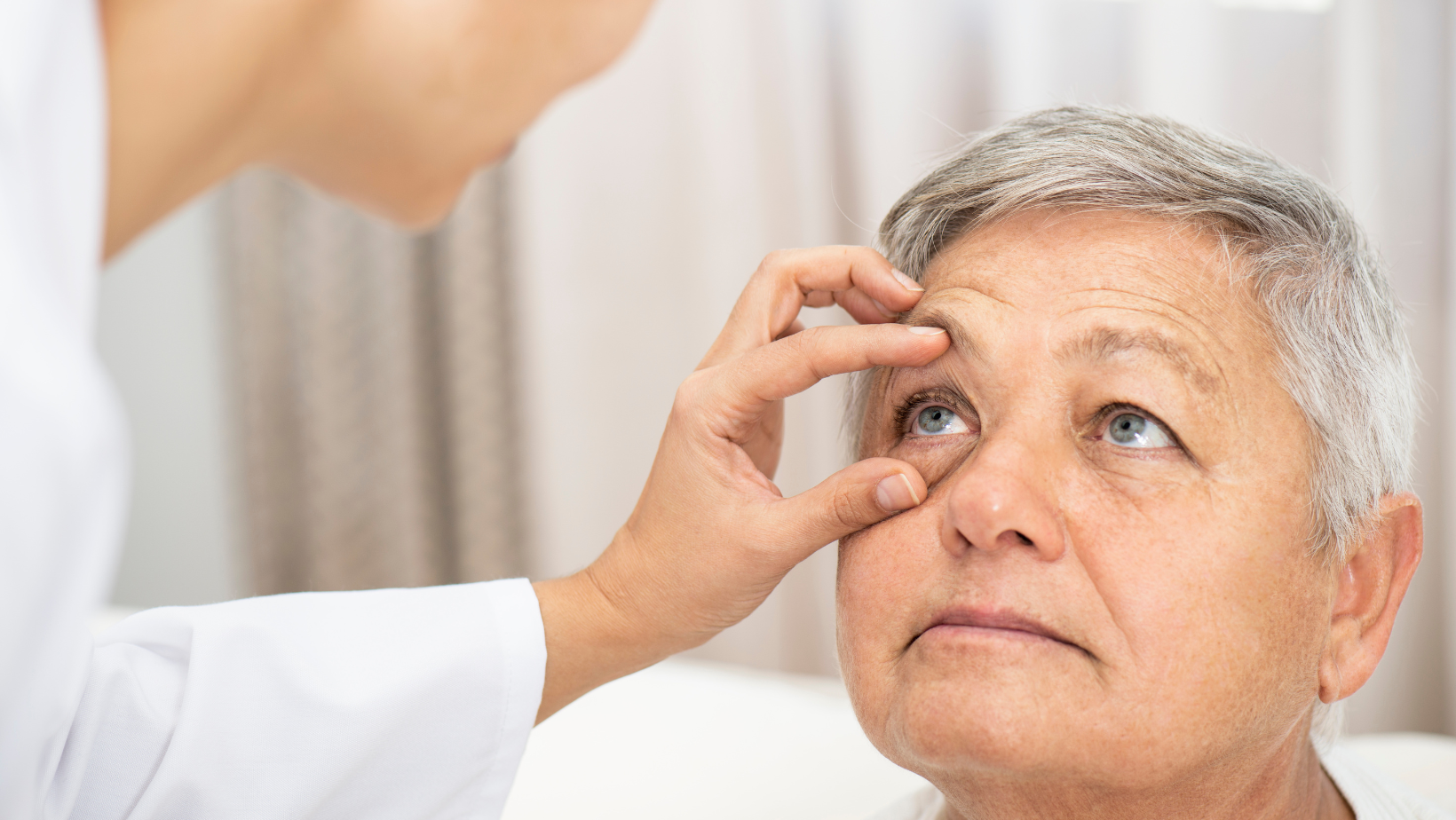
A baseline ophthalmic exam is required for patients and exclude those patients with active conditions on surface of eye, such as cicatricial conjunctivitis, thermal/chemical burns, Stevens Johnson syndrome or infectious conjunctivitis. Another exam is required within 14 days of the first dose of an antibody-drug conjugate. A swift referral (<72 hours) of patients to an ophthalmologist should occur upon an ocular adverse event of any grade. They should continue to see an ophthalmologist until symptoms resolve.
In addition, primary prophylaxis is necessary to avoid certain ocular toxicities. For example, prophylactic use of preservative-free lubricating eye drops is required for daily use throughout treatment and 30 days after the final dose of tisotumab-vedotin. The use of lubricant eye drops is also required for mirvetuximab-soravtansine treated patients.
For tisotumab-vedotin, corticosteroids (1 drop of dexamethasone 0.1% per eye from the day before until day 3 of the cycle) and vasoconstrictors (3 drops of brimonidine tartrate 0.2% before each infusion) are part of the treatment protocol. Cooling pads are also recommended during the infusion, but contact lenses are not allowed throughout treatment.
For mirvetuximab-soravtansine, corticosteroids are also part of the treatment protocol. Patients should apply 1 drop of steroids (prednisolone 1%, difluprednate 0.5%) to each eye six times each day before the cycle begins until the 4th day. On the 5th to the 8th day of each treatment cycle, patients should apply 1 drop of steroids into each eye four times daily. A warm compress before sleep can help reduce inflammation. Cleansing with baby shampoo and a soft eye cloth may also be done daily. Contact lenses are not allowed in patients treated with mirvetuximab-soravtansine.
There are other important considerations to manage patients receiving antibody-drug conjugates. Most grade 3-4 ocular toxicities experienced by patients treated with tisotumab-vedotin necessitates permanent discontinuation of the drug. In addition, any grade of conjunctival or corneal scarring or symblepharon necessitate permanent discontinuation.
Mirvetuximab-soravtansine has more complicated management protocols. Dose reductions, treatment holds, and treatment times govern the management guidelines. Datopotamab deruxtecan has simpler management protocols. This agent’s treatment is withheld at grade 2 and grade 3 until the symptoms improve and revert back to grade 1. Datopotamab deruxtecan is permanently discontinued at grade 4.
Conclusions
Antibody-drug conjugates are a welcome inclusion in oncology formularies. Similar to prophylactic treatment to prevent nausea, vomiting, or kidney damage before administering certain chemotherapies, these agents require a prophylactic plan for eye care. Establishing a collaborative relationship between oncologists, pharmacists, and ophthalmologists among practices that incorporate antibody-drug conjugates is also very important. Educating patients and their caregivers before treatment begins is also vital. These guidelines and management techniques can help alleviate concerns with the clinical administration of antibody-drug conjugates and patient adherence to treatment. Overall, this will improve outcomes in oncology care.
Reference:
This article is based on the publication, “Practical clinical management of ocular adverse events related to Antibody-Drug Conjugates in gynaecological malignancies” by Zinna BB, Rousseau F, Fauquier S, Sabatier R and Kfoury M. It was published in Cancer Treatment Reviews on December 21, 2024.