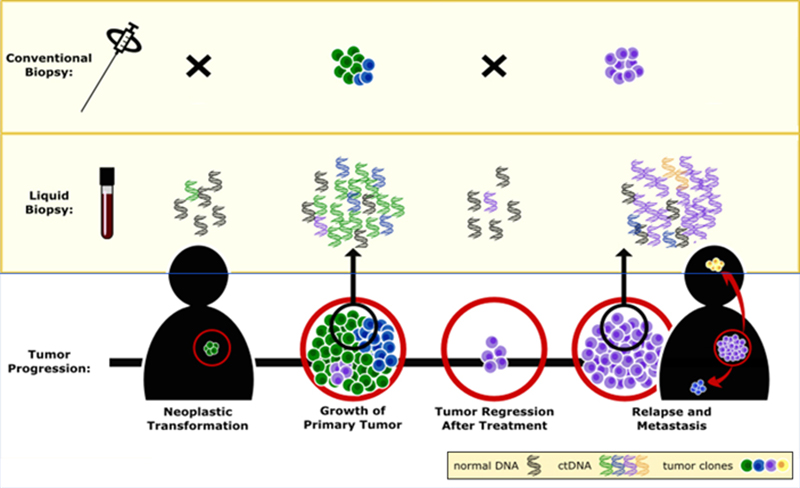
CASE SCENARIOS SHOWING APPLICATIONS OF ctDNA MONITORING
Because the ctDNA test enables more sensitive detection of response as compared with other biomarkers or imaging scans, it enables earlier informed treatment decisions. Potential clinical scenarios to illustrate its utility include the following:
Selection of an Aggressive Combination vs Single-Agent Approach
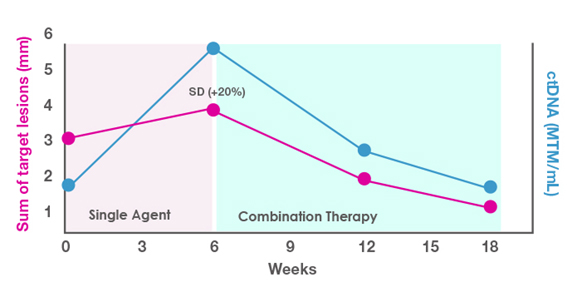
You are evaluating a 65-year-old man with lung cancer and multiple comorbidities for single-agent vs combination therapy in the first-line setting. You decide to use the single-agent approach and monitor the response with ctDNA. As shown in Figure 2, at 6 weeks you see a rise in ctDNA levels and a slight increase in tumor measurements (stable disease). Based on this lackluster response, you decide to add a second agent to the therapy. In this situation, the combination approach was effective, as shown in the drop in both ctDNA levels and target lesion measurements after the second agent was added.
Figure 2. ctDNA-guided decision making for a patient with stable disease (SD).
Determining the Treatment Course When Imaging Results Are Difficult to Interpret
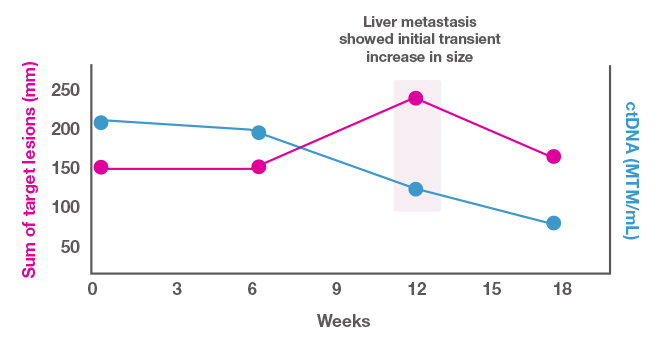
You are following a 42-year-old woman with liver metastases from triple-negative breast cancer who is receiving immunotherapy. As shown in Figure 3, the target lesion appears to be growing, but the ctDNA level is dropping. You suspect that the lesion expansion is pseudoprogression, characterized by an initial increase in the size of a tumor or new lesion formation during the early stages of checkpoint inhibitor therapy.[Jia 2019] Given the drop in the ctDNA levels, you feel confident in keeping the patient on the current therapy. This decision appears correct, since both the target lesion size and ctDNA levels drop thereafter.
Figure 3. Role of ctDNA in treatment decision making in a patient with pseudoprogression.
Determining Whether Therapy Can Be Stopped
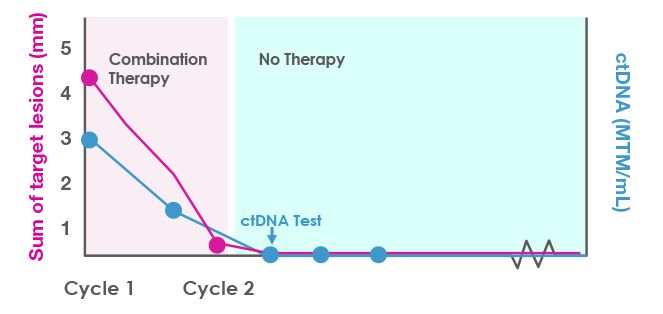
After obtaining a baseline ctDNA level, you treat a 35-year-old woman who has aggressive Stage IV melanoma with combination checkpoint inhibitor therapy. She completes 2 cycles but gets very sick with immune-related adverse effects, and you discontinue therapy. However, her scans look much improved after the discontinuation, as shown in Figure 4. At the next ctDNA check, her level is 0 MTM/mL. The clearance of the ctDNA is consistent with a molecular complete response. You decide not to reinitiate therapy and just monitor her at this point. The next few ctDNA tests continue to show clearance, suggesting a durable response.
Figure 4. ctDNA monitoring to clarify the duration and depth of response in a patient with a strong early response to combination immunotherapy.
ctDNA TESTING DETAILS
Testing Requirement
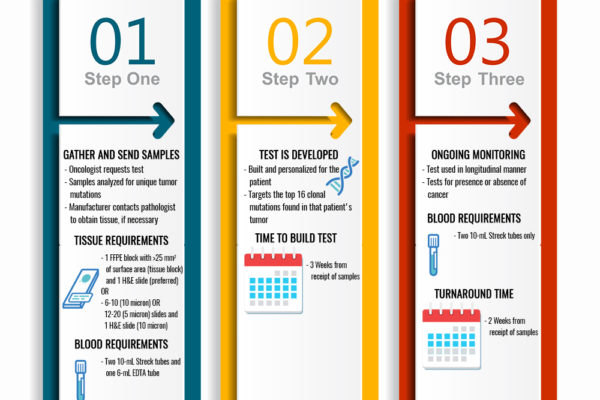
Figure 5 shows the testing process, timing, and blood and tissue requirements for ctDNA.
Figure 5. Testing process and blood and tissue requirements with the ctDNA test. FFPE = formalin-fixed paraffin embedded; H&E = hematoxylin & eosin; EDTA = ethylenediamine tetraacetic acid.
Billing and Coverage
- Medicare: covered
- Commercial insurance: manufacturer will work with the various commercial insurances
- Cash pay rate
- Compassionate Care program for uninsured patients or those concerned about their ability to pay (contact signaterapc@natera.com)
- Contact for billing information: 415-918-6329
Patient Education
RESEARCH APPLICATION
The BESPOKE IO trial is a clinical research study, currently in recruitment stages, which is evaluating clinical outcomes when ctDNA is integrated into a checkpoint inhibitor treatment paradigm for colorectal cancer, melanoma, and non-small cell lung cancer [Natera 2021; clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT04761783)]
REFERENCES:
Abbosh C, Birkbak NJ, Wilson GA, et al; on behalf of the TRACERx and PEACE consortia. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545:446-451.
Bratman SV, Yang SYC, Iafolla MAJ, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020;1:873–881.
Jia W, Gao Q, Han A, Zhu H, Yu J. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med. 2019;16:655-670.
Lee JH, Menzies AM, Carlino MS, et al. Longitudinal monitoring of ctDNA in patients with melanoma and brain metastases treated with immune checkpoint inhibitors. Clin Cancer Res. 2020;26:4064-4071.
Natera, Inc. BESPOKE Study of ctDNA Guided Immunotherapy. Austin, TX: Natera, Inc. https://www.clinicaltrials.gov/ct2/show/NCT04761783. Accessed September 20, 2021.
Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11, 3801. https://doi.org/10.1038/s41467-020-17670-y
Seremet T, Jansen Y, Planken S, et al. Undetectable circulating tumor DNA (ctDNA) levels correlate with favorable outcome in metastatic melanoma patients treated with anti-PD1 therapy. J Transl Med. 2019;17:303. doi: 10.1186/s12967-019-2051
Zhang Y, Yao Y, Xu Y, et al. Pan-cancer circulating tumor DNA detection in over 10,000 Chinese patients. Nat Commun. 2021;12(1):11. doi: 10.1038/s41467-020-20162-8
Acknowledgment: We thank Natera for an unrestricted educational grant in support of this primer.