- Introduction
- What is oncolytic virus therapy or oncolytic virotherapy?
- Which viruses are used for oncolytic virus therapy and are they natural?
- Are there any FDA-approved oncolytic virotherapies?
- Are there any oncolytic virotherapies in clinical trials?
- Are oncolytic viruses dangerous to the patient and those around them?
- What types of cancer can be treated with oncolytic virotherapy?
- How successful is oncolytic virus therapy?
Oncolytic Viruses
Introduction
The skin covering our bodies is our first line of immune defense, acting as a natural barrier. When everything works normally, the immune system is active within our skin and is our second line of defense. The immune system protects us from infection and disease, but sometimes it is prevented from doing its job.
Skin cancers can develop when tumor cells shut off the immune system’s response or when someone has an immune system that has been weakened by disease or medication. If the immune system is off, it does not kill cancerous cells and tumor development can continue. Immunotherapy (meaning “immune” + “therapy”) drugs turn the immune system’s response back on.
The immune system usually is present and vigilant within our skin. The constant vigilance helps explain why skin cancers are often responsive to immunotherapy drugs. Re-energizing immune system activity can result in an immune cell’s ability to fight cancer and kill tumor cells.
Before 2011, there were few options to treat patients with melanoma effectively. Immunotherapies like atezolizumab (Tecentriq), cemiplimab (Libtayo), ipilimumab (Yervoy), nivolumab (Opdivo), and pembrolizumab (Keytruda) have revolutionized the treatment of many cancers, including skin cancer. Cancer cells evade our immune system attack by displaying certain molecules on their surface that make them invisible to the immune system. Immunotherapy removes these molecules and allows our immune system to identify and attack cancer cells.
Drugs like nivolumab and pembrolizumab belong to a specific group of immunotherapies called immune checkpoint inhibitors to describe their specific action. Immune checkpoint inhibitors work well for many patients with skin cancer, but not everyone will experience a dramatic improvement. Here we will explain a different type of immunotherapy called oncolytic virus therapy or oncolytic virotherapy. Though oncolytic virotherapy differs from immune checkpoint inhibitors, these drugs are categorized broadly as immunotherapies.
What is oncolytic virus therapy or oncolytic virotherapy?
Oncolytic virotherapy is a treatment that uses a human virus modified in a laboratory to target and infect cancer cells while leaving most normal cells alone. After infection, the virus multiplies itself within the cancer cell. Eventually, the cancer cell will die and burst open. This outcome releases viruses around the area to infect neighboring tumor cells.
The virus is also modified with components to stimulate an immune response. When the infected cancer cell opens and releases its contents, immune cells are alerted and promote activation of the immune system. Essentially, this re-energizes the body to attack cancer using its immune system.
Video of Jose Lutzky: What is oncolytic virus therapy?
Which viruses are used for oncolytic virus therapy and are they natural?
Herpes Simplex Virus Type 1 or HSV-1 is the virus that has been used with the most success. HSV-1 is a common virus that causes cold sores. The HSV-1 strain used for therapy was initially taken from a human volunteer with a herpes cold sore. Then, it was modified in a laboratory to be a weaker form of the natural virus to prevent infecting non-cancer cells. The modified virus is the one used to make the drug. It is also engineered with new properties to enhance activity against cancer.
Besides the herpes virus, researchers and pharmaceutical companies have tested many other types of viruses. Viruses are being exploited as oncolytic virotherapy, cancer vaccines, or vaccines for other diseases in ongoing clinical trials. Because of the need for vaccine development during the COVID-19 pandemic, there is enormous interest in modifying viruses for therapy. The FDA-approved COVID-19 vaccines manufactured by AstraZeneca and Johnson & Johnson use viruses.
Some viruses such as the Coxsackie virus A21 have a natural tendency to preferentially infect cancers, such as melanoma, by attaching to receptors that are displaying the cancer cells. However, in nonmelanoma skin cancers, there are no approved viruses for use in virotherapy.
Video of Jose Lutzky: Which oncolytic viruses are the most useful and are they natural?
Are there any FDA-approved oncolytic virotherapies?
Yes, there is an oncolytic virotherapy drug used for melanoma treatment. Imlygic® (talimogene laherparepvec) or T-VEC was approved in 2015 for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma that returns after surgery. This therapy is a weakened virus engineered to stimulate the immune system injected directly into the tumor lesion.
T-VEC is used on local skin lesions and is not intended to shrink metastatic tumors elsewhere. The method of oncolytic virotherapy delivery is called intralesional or intratumoral injection. It means that the drug reaches the tumor through a needle injection. The technique is different from the way other types of drugs are given (Figure 1).
In 2022, the FDA also approved Adstiladrin® (nadofaragene firadenovec), a non-oncolytic virotherapy to treat non-muscle invasive bladder cancer that is unresponsive to treatment with bacillus Calmette Guerin therapy. It is called non-oncolytic because the virus does not work by directly destroying the tumor cell after infection. This therapy works differently.
There are no FDA approvals for oncolytic virotherapy in nonmelanoma skin cancer.
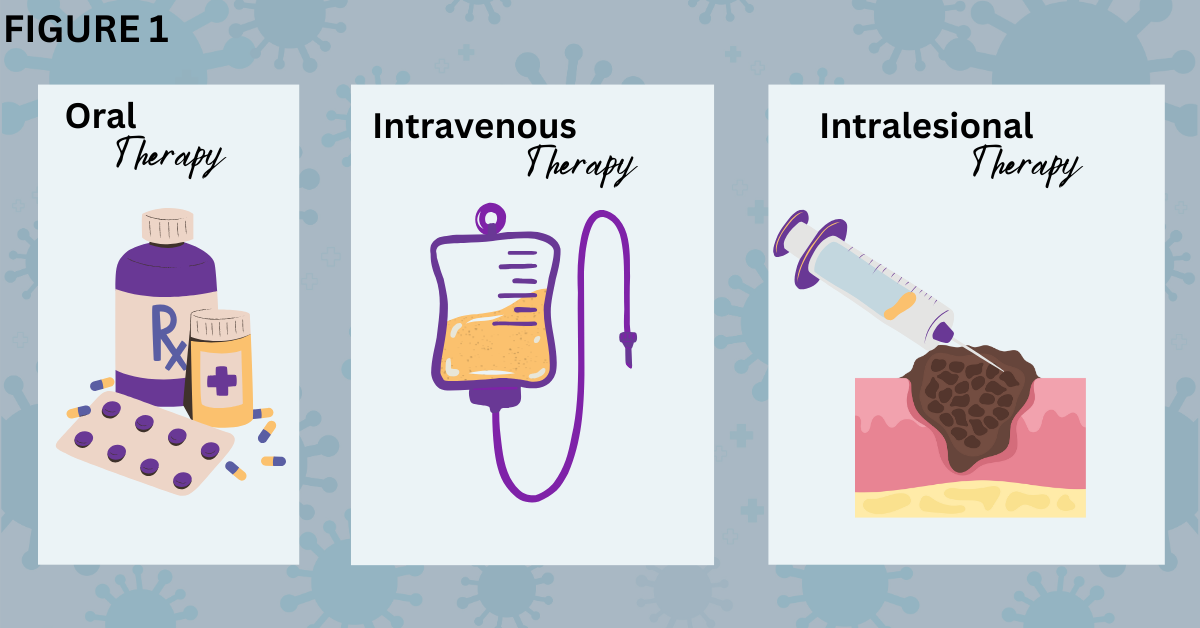
Figure 1. Comparison of different methods for delivering cancer drugs.
Some targeted inhibitors like trametinib (Mekinist®), vemurafenib (Zelboraf®), and vismodigeb (Erivedge®), are prescribed to patients in pill form to swallow (left). This is called oral drug therapy because it goes through the mouth and is then digested by the body. Patients take this type of medication on a schedule at home or outside a medical facility. Intravenous therapy is delivered into a patient’s body via injection into a vein (middle). Immunotherapies like cemiplimab (Libtayo®), ipilimumab (Yervoy®), nivolumab (Opdivo®), and pembrolizumab (Keytruda®) are given intravenously. (Traditional chemotherapy is also given intravenously for many cancers, but melanoma does not generally respond to chemotherapy.) A clinician introduces an intravenous line, or the patient may have a port through which the drug is dispensed. This type of therapy occurs in a doctor’s office, hospital, or medical facility that monitors the patient during the intravenous infusion of the drug. Intralesional injected therapy (right), which may also be called intratumoral injection, is given using a needle to inject the oncolytic virotherapy directly into the melanoma tumor lesion. T-VEC or talimogene laherparepvec (Imlygic®) is an example of this therapy. BioRender was used to create the image.
Video of Jose Lutzky: What oncolytic viruses are FDA approved?
Are there any oncolytic virotherapies in clinical trials?
Yes, oncolytic virotherapies are in clinical trials for many types of cancers, including nonmelanoma skin cancer. The oncolytic virotherapies may be tested alone, but often they are combined with immune checkpoint inhibitors. The combination appears to make the virotherapy work better.
For example, a recent clinical trial of oncolytic virotherapy called vusolimogene oderparepvec (RP1) was tested in combination with cemiplimab (Libtayo®), which is an approved immunotherapy drug. The clinical trial included patients diagnosed with locally advanced or metastatic cutaneous squamous cell carcinoma, sometimes referred to as squamous cell skin cancer.
In this trial, 120 patients with squamous cell skin cancer received an intralesional injection of vusolimogene oderparepvec (RP1) and cemiplimab (Libtayo®). Among the patients who received the drug combination, 48.1% had a complete response. Of the patients receiving only cemiplimab (Libtayo®), 22.6% had a complete response. Although there was better response in patients who received the oncolytic virotherapy in this trial, further follow-up is required for this therapy, and it is not currently approved by the FDA.
Many types of viruses are being tested for vaccines or oncolytic virotherapy in ongoing clinical trials. Besides the herpes virus, adenoviruses and echoviruses have succeeded developing oncolytic virotherapy and vaccines. Other types under investigation include coxsackie viruses, lentivirus, measles, poliovirus, reoviruses, vaccinia viruses, and vesicular stomatitis viruses.
Are oncolytic viruses dangerous to the patient and those around them?
There is some risk with administering, receiving, and transmitting oncolytic viruses related to therapy, but attention to safety instructions should be sufficient to protect yourself and your family.
T-VEC is a live, weakened herpes simplex virus that can cause life-threatening disseminated herpetic infection in patients who are immunocompromised. Health care providers are directed to avoid direct contact with injected lesions, dressings, or body fluids of treated patients.
Immunocompromised or pregnant individuals should not prepare or administer T-VEC. Exposed individuals should clean the affected area. Personal protective equipment is required during preparation, including a face shield. Wounds must be covered before handling the agent. Accidental exposure may lead to transmission and herpetic infection.
The spread of a herpes infection in the body is possible among treated patients. The virus will appear among treated patients in blood, urine, saliva, and the injection site. The HSV-1 virus can spread to those with whom patients have close contact. Engaging in kissing with an open sore or sexual activity could spread the virus. Touching the eyes after manipulating the injected wound could lead to herpetic keratitis, a serious viral infection of the cornea that can potentially result in visual loss.
Because therapies like T-VEC contain an active virus, it is not recommended for use in pregnant or immunocompromised patients such as organ transplant patients or those infected with HIV. If patients treated with T-VEC have close contact with someone who has a weakened immune system or may be pregnant, their physician should be informed. It is also important to know all the medications that the treated patient is taking, as some could interfere with how the drug is intended to work.
Further, it is recommended to wear gloves when changing injected tumor wound coverings. The treated lesions should be covered for at least one week after injection with air- and water-tight bandages. The used bandages that covered the treated site should be disposed of in a sealed plastic bag before placing them into the trash. Alternatively, patients may be provided with biohazard bags to take home for disposal of used dressings (Figure 2). When filled with used dressings, these bags should be brought back to the clinic for disposal at your next visit.
Video of Jose Lutzky: Are oncolytic viruses dangerous to the patient and those around them?
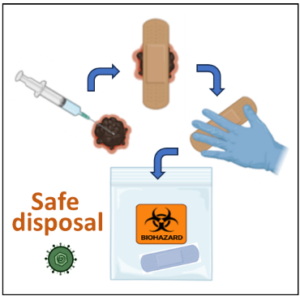 Figure 2. Safe disposal of wound coverings after treatment injection with T-VEC or talimogene laherparepvec (Imlygic®).
Figure 2. Safe disposal of wound coverings after treatment injection with T-VEC or talimogene laherparepvec (Imlygic®).
Each site where intralesional injection occurred should be covered for 5-7 days after treatment with a bandage or dressing, one that is air- and water-tight. These wound coverings or dressings may have modified herpes virus particles on them. Gloves should be used when changing the coverings to keep others safe from the virus. The discarded materials should be placed into a plastic or biohazard bag for disposal. Once complete, the biohazard bag should be returned to the clinic on your next visit. The facility can safely discard these materials. BioRender was used to create the image.
What are some common side effects of these treatments?
Fatigue is the most common side-effect, occurring in more than half of treated patients. Chills, fever, influenza-like illness, injection site pain, and nausea may also occur several hours after injection. In clinical trials, patients treated with T-VEC reported mild or moderate adverse reactions that generally resolved within 72 hours.
Tumor killing by the virus may result in open wounds that could become infected with bacteria causing cellulitis and even more widespread infections. Auto-immune-like responses are a risk with treatment, albeit not frequent.
Video of Jose Lutzky: What are side effects of oncolytic virus therapy?
How do oncolytic virotherapies kill cancer cells?
The overall process provokes an immune response against cancer cells in the injected tumor. Although the mechanism is not fully understood, virotherapy uses the modified virus’s programming to copy itself repeatedly inside tumor cells. It hijacks the tumor cell’s resources, turning it into a virus-manufacturing factory. Eventually, the cell runs out of resources.
The dysfunction caused by the virus can lead to tumor cell death and rupture of the tumor cell (Figure 3). The contents spilled out are recognized by the immune system as alarms. Other contents released from the tumor cells are proteins that will stimulate the production of immune cells. The new immune cells will rush into the area and engage with neighboring tumor cells, killing them.
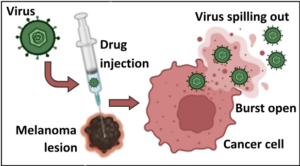 Figure 3. The oncolytic virus causes death and rupture of the tumor cell.
Figure 3. The oncolytic virus causes death and rupture of the tumor cell.
The HSV-1 virus is modified for use in the drug T-VEC. It is injected into the melanoma tumor lesion. The cancer cell begins to manufacture many viruses, using up its resources. Eventually, it will die and burst open, spilling virus out of the cell to neighboring cells. Although the cancer cell is dead, the immune system is alerted by the virus particles and becomes active. The immune activation leads to the additional killing of other cancer cells. BioRender was used to create the image.
Video of Jose Lutzky: How do oncolytic viruses kill cancer cells?
What types of cancer can be treated with oncolytic virotherapy?
T-VEC was approved in the U.S. in 2015 for the treatment of melanoma. Clinical trials with oncolytic virotherapies for nonmelanoma skin cancer and Merkel cell carcinoma are also ongoing. The website https://clinicaltrials.gov can provide additional information about enrollment status for trials.
In countries outside of the U.S., oncolytic virotherapy is used in combination with chemotherapy and/or radiation therapy for the treatment of glioblastoma and nasopharyngeal carcinoma. In addition, phase 2 clinical trials have investigated oncolytic virotherapy against bladder, colorectal, lung, and pancreatic cancer. However, oncolytic virotherapies to treat these cancers are not approved to date.
Video of Jose Lutzky: What types of cancer can be treated with oncolytic virus therapy?
How successful is oncolytic virus therapy?
The final analysis of clinical trial results that led to the approval of T-VEC in melanoma showed a durable response rate of 19% for patients who received T-VEC. A durable response was defined as shrinkage of the tumor that lasted for at least 6 months. Among the patients who received a control treatment, the durable response rate was 1.4%.
More impressive results were reported in a European clinical trial among patients with Stage III melanoma and Stage IV M1a melanoma (M1a means the tumor has metastasized to distant skin, soft tissue including muscle, and/or to distant lymph nodes). The patients receiving treatment with T-VEC had an overall survival of 46.8 months. Among patients in the control arm, the overall survival time was 21.5 months.
Studies are ongoing for approvals in nonmelanoma skin cancer. To date, there are no approvals of oncolytic virotherapy in nonmelanoma skin cancer.
Video of Jose Lutzky: Does oncolytic virus therapy work?
References
Adstiladrin® (nadofaragene firadenovec) [package insert]. Kastrup, Denmark: Ferring Pharmaceuticals: 2022.
Chen L, Zuo M, Zhou Q, et al. Oncolytic virotherapy in cancer treatment: challenges and optimization prospects. Front Immunol. 2023;14: 1308890. doi: 10.3389/fimmu.2023.1308890.
Replimune [press release]. https://ir.replimune.com/news-releases/news-release-details/replimune-shares-initial-primary-analysis-results-cerpass. December 5, 2023.
Shalhout SZ, Miller DM, Emerick KS, et al. Therapy with oncolytic viruses: progress and challenges. Nat Rev Clin Oncol. 2023;20(3):160-177. doi: 10.1038/s41571-022-00719-w.
Thomas S, Kuncheria L, Roustone V, et al. Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simple virus type 1. J Immunother Cancer. 2019;7(1):214. doi: 10.1186/s40425-019-0682-1.
Trivedi PD, Byrne BJ, Corti M. Evolving Horizons: Adenovirus Vectors’ Timeless Influence on Cancer, Gene Therapy and Vaccines. Viruses. 2023;15(12):2378. doi: 10.3390/v15122378.
IMLYGIC® (talimogene laherparepvec) [package insert]. Thousand Oaks, California: Amgen: 2015.
Wongariyapak A, Roulstone V, Melcher AA, et al. Combination strategies incorporating oncolytic viruses and immune checkpoint inhibitors for advanced melanoma: what is the evidence? Ann Transl Med. 2023;11(10):369. doi: 10.21037/atm-2023-5.
